Pediatric COVID-19 Vaccines Recommended for Children Aged 6 mo – 5 years
Background:
On June 15, 2022, the Vaccines and Related Biological Products Advisory Committee (VRBPAC) unanimously voted to recommend the authorization of both the Moderna COVID-19 vaccine two-dose primary series for children 6 months to 5 years of age and the Pfizer three-dose series for children 6 months to 4 years of age under Emergency Use Authorization (EUA). On June 17, 2022, the U.S. Food and Drug Administration (FDA) authorized the emergency use of the Moderna COVID-19 vaccine and the Pfizer-BioNTech COVID-19 vaccine for the prevention of COVID-19 to include use in children under 5 years of age.
According to disease burden data presented on day one of the two-day Advisory Committee on Immunization Practices (ACIP) meeting, children ages 6 months through 4 years are at risk of severe COVID -19 infection and more than 2 million cases of the illness have been identified among this population. COVID-19-associated hospitalizations among children ages 6 months through 4 years have similar severity compared to older children and adolescents and more than half of the hospitalized children ages 6 months through 4 years had no underlying conditions. The burden of COVID- 19associated death among this population is similar to or exceeds that of other pediatric vaccine-preventable diseases. On June 18, 2022, ACIP unanimously voted to recommend the use of the Moderna COVID-19 vaccine two-dose primary series for children ages 6 months to 5 years and the Pfizer three-dose series for children ages 6 months to 4 years.
Recommendations for Non-Immunocompromised Children
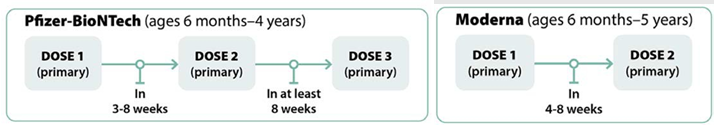
Recommendations for Moderately to Severely Immunocompromised Children
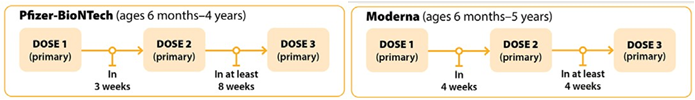
In general, the same mRNA vaccine product should be used for all doses in the primary series. COVID-19 vaccines may be co-administered with other routine age-appropriate vaccines at the same visit. Prior infection may not provide broad protection against newer SARS-CoV-2 variants and vaccination should still be recommended post infection. For children who have been infected with COVID-19, their next dose can be delayed three months from when symptoms started or, if they did not have symptoms, when they received a positive test. For additional information regarding the definition of what is included with moderate to severe immunocompromising conditions, timing and interchangeability of COVID-19 vaccines, refer to the interim clinical considerations found here.
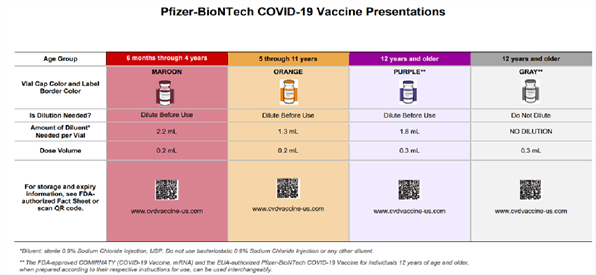
Pfizer-BioNTech’s vaccine information fact sheets for recipients and/or caregivers and healthcare providers have also been updated by the FDA for reference, in addition to a Letter to Healthcare Providers and a comprehensive wall chart (also see screen shot below).
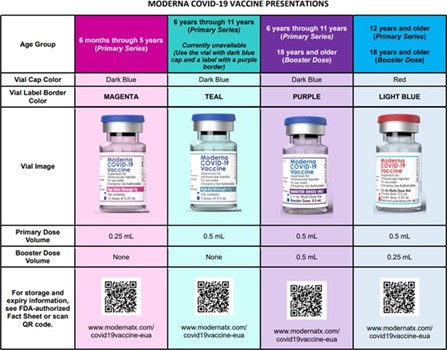
Moderna’s vaccine information fact sheets for recipients and/or caregivers and healthcare providers have also been updated by the FDA for reference, in addition to a comprehensive wall chart (see screen shot below).
After careful review of the data presented to the U.S. FDA VRBPAC meeting on June 14-15, 2022, and to the ACIP meeting held on June 17-18, 2022, and each committee’s recommendations concerning the Moderna COVID-19 vaccine for infants and children 6 months through 5 years of age (two vaccine doses) and the Pfizer BioNTech COVID-19 vaccine for infants and children 6 months through 4 years of age (three vaccine doses), the Western States Scientific Safety Review Workgroup concluded that the benefits of completing the COVID-19 vaccine series substantially outweigh the known or likely risks. “The Workgroup strongly encourages our states to strengthen existing efforts to ensure widespread, equitable access to age-appropriate COVID-19 vaccines among all vaccine-eligible individuals who have not yet been vaccinated or boosted, regardless of geographic location, socio-economic status, race and ethnicity, and immigration status. We must assure that all our states’ diverse communities are aware that now everyone over 6 months of age is recommended to receive COVID-19 vaccines.” The Workgroup also encourages, “All healthcare providers and vaccine recipients to report any suspected adverse events following receipt of a COVID-19 vaccine to the Vaccine Adverse Events Reporting System (VAERS) and vaccine recipients to participate in the V-safe system because the various U.S. systems for monitoring the safety of vaccinations, including COVID-19 vaccines are important to sustaining confidence in immunization and guiding vaccination policy.”
The Nevada Department of Health and Human Services is encouraging families to speak with a health care provider about vaccination and COVID-19 vaccines. Families may be referred to NVCOVIDFighter.org or 800-401-0946 for more information on vaccine access and other COVID-19 resources.
It is important to note the primary goal of the COVID-19 vaccine response should continue to be COVID-19 vaccine administration to the unvaccinated.
Questions:
For updated guidance, review the DPBH Technical Bulletin web page and the Nevada Health Response website regularly. Email questions to dpbhcovid19vax@health.nv.gov.

