Hantavirus in Nevada
Summary
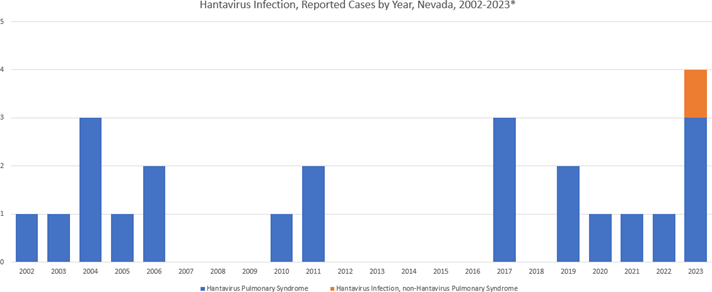
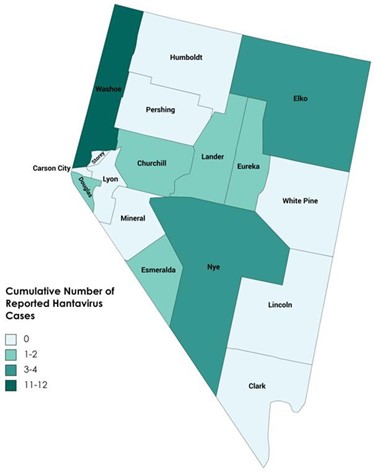
To date in 2023 there have been four (4) cases of hantavirus reported in Nevada. One case was hantavirus infection, Non-Hantavirus Pulmonary Syndrome in Washoe County; and three (3) were Hantavirus Pulmonary Syndrome (HPS) with two (2) cases in Washoe County and one (1) case in Churchill County.
Figure 1. Hantavirus Infection, Reported Cases by Year, Nevada, 2002-2023*
Note: The information in this technical bulletin is compiled directly from the American Academy of Pediatrics 2021 RedBook and the American Public Health Association’s Control of Communicable Diseases Manual (21st Edition). Minimal changes were made to preserve the complex details in these references.
Figure 2. Cumulative Number of Reported Hantavirus Cases by County, Nevada, 2002-2023*
Background
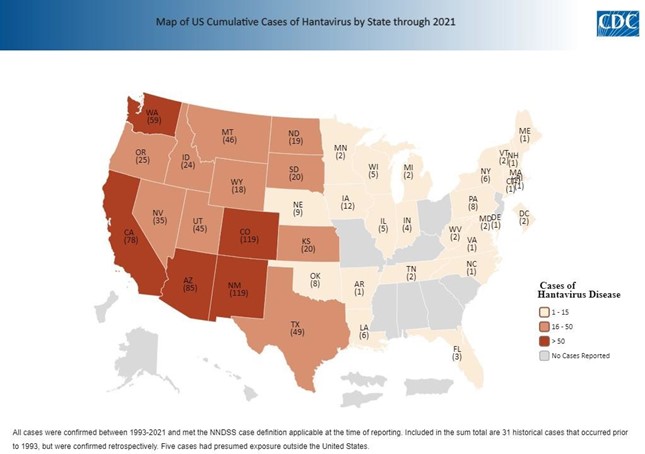
Hantaviruses are RNA viruses of the Hantaviradae family. Sin Nombre virus (SNV) is the major cause of HPS in the western and central regions of the United States (including Nevada). There are typically 20 to 40 cases of HPS reported annually in the United States, with the majority (>95%) of cases occurring west of the Mississippi River.
The clinical syndrome of HPS was first recognized in 1993 and has since been identified throughout the United States.
HPS is an acute febrile illness (i.e., temperature greater than 101.0 F) with a prodrome consisting of fever, chills, myalgia, headache and gastrointestinal symptoms with:
- bilateral diffuse interstitial edema, or
- acute respiratory distress syndrome (ARDS), or
- radiographic evidence of noncardiogenic pulmonary edema.
In a small proportion of patients with hantavirus infection, cardiopulmonary symptoms do not develop. These patients are considered to have hantavirus infection Non-Hantavirus Pulmonary Syndrome.
Non-HPS Hantavirus Infection is a febrile illness with non-specific viral symptoms including fever, chills, myalgia, headache and gastrointestinal symptoms, but no cardiopulmonary symptoms.
Figure 3. Cumulative Hantavirus Cases by State, U.S., 1992-2021
Reservoir
The major reservoir of Sin Nombre virus in North America (and in Nevada) is the deer mouse (Peromyscus maniculatus). Antibodies have also been found in other Peromyscus spp., including pack rats, chipmunks and other rodents. Past surveillance has indicated about 17% of deer mice were infected with hantavirus in Washoe County; however, regular testing was discontinued in 2013.
Transmission
Rodents are natural hosts for hantaviruses and acquire lifelong, asymptomatic, chronic infection with prolonged viruria and virus in saliva and feces. Humans acquire infection through direct contact with infected rodents, rodent droppings, or rodent nests or through the inhalation of aerosolized virus particles from rodent urine, droppings, or saliva. Rarely, infection may be acquired from rodent bites or contamination of broken skin with excreta. At-risk activities include handling or trapping rodents; cleaning or entering closed or rarely used rodent-infested structures; cleaning feed storage or animal shelter areas; hand plowing; and living in a home with an increased density of mice.
For backpackers or campers, sleeping in a structure inhabited by rodents has been associated with HPS, with a notable outbreak occurring in 2012 in Yosemite National Park secondary to rodent-infested cabins. Exceptionally heavy rainfall improves rodent food supplies, resulting in an increase in the rodent population with more frequent contact between humans and infected rodents, resulting in more human disease. Most cases occur during the spring and summer.
Signs and Symptoms
After an incubation period of 1 to 6 weeks, the prodromal illness of HPS lasts 3 to 7 days and is characterized by fever, chills, headache, myalgia, nausea, vomiting, diarrhea, dizziness and sometimes cough. Respiratory tract signs or symptoms usually do not occur during the first 3 to 7 days, but then pulmonary edema and severe hypoxemia appear abruptly and present as cough and dyspnea; the disease then progresses over hours. In severe cases, myocardial dysfunction causes hypotension, which is why the syndrome sometimes is called hantavirus cardiopulmonary syndrome.
The mortality rate for patients with HPS is between 35% and 50%; death usually occurs in the first 1 or 2 days of hospitalization.
Guidance for Health Care Providers
Diagnosing HPS in an individual who has only been infected a few days is difficult because early symptoms such as fever, muscle aches and fatigue are easily confused with other illnesses such as influenza. However, if the individual is experiencing fever, fatigue and shortness of breath with a history of potential rodent exposure, this would be strongly suggestive of HPS.
Clinical Findings
HPS should be considered when thrombocytopenia occurs with severe pneumonia clinically resembling acute respiratory distress syndrome in the proper epidemiologic setting. Other characteristic laboratory findings include neutrophilic leukocytosis with immature granulocytes, including more than 10% immunoblasts (basophilic cytoplasm, prominent nucleoli, and an increased nuclear-cytoplasmic ration) and increased hematocrit.
In areas where HPS is known to occur, use of a 5-point peripheral blood screen has aided in the early detection of patients with HPS. Elements of the screen are:
- hemoglobin elevated for gender/age;
- left shift of granulocytic series;
- absence of toxic changes;
- thrombocytopenia; and
- immunoblasts and plasma cells >10% of lymphocytes.
For cases fulfilling 4 of 5 criteria, the positive predictive value of the 5-point screen is >90%.
Diagnosis and Laboratory Confirmation
Serologic testing remains the method of choice for diagnosis. Immunoglobulin class M (IgM) and immunoglobulin class G antibodies (IgG) (detectable by enzyme-linked immunosorbent assay, or ELISA) often are present at the onset of clinical disease. The Nevada State Public Health Laboratory can perform the hantavirus (Sin Nombre virus) IgG and IgM ELISA on serum or blood clot specimens as well as lung tissue from autopsy (https://med.unr.edu/public-health-lab/about/clinical-analysis/hantavirus-igg-and- igm). If positive results are found, reverse transcription polymerase chain reaction (RT-PCR) amplification will be performed automatically (https://med.unr.edu/public-health-lab/about/clinical-analysis/hantavirus- rt-pcr).
Virus levels will have declined at the time of hospitalization so virus isolation via culture is not usually possible.
Clinicians can contact the CDC’s Viral Special Pathogens Branch Clinical Inquiries Line (470-312-0094) for assistance with diagnosis and management of hantavirus cases.
Treatment
Patients with suspected HPS should be transferred immediately to a tertiary care facility where supportive management of pulmonary edema, severe hypoxemia and hypotension can occur during the first critical 24 to 48 hours.
In severe forms, early mechanical ventilation and inotropic and pressor support are necessary. Extracorporeal membrane oxygenation (ECMO) should be considered when pulmonary wedge pressure and cardiac indices have deteriorated and may provide short-term support for the severe capillary leak syndrome in the lungs.
Ribavirin is active in vitro against hantaviruses, including SNV. However, two clinical studies of intravenous ribavirin (one open-label study and one randomized, placebo-controlled, double-blind study) failed to show benefit in treatment of HPS in the cardiopulmonary stage. At the present time, ribavirin should not be considered the standard of care.
Cytokine-blocking agents for HPS theoretically may have a role, but these agents have not been evaluated in a systematic fashion. Antibacterial agents are unlikely to offer benefit. However, broad- spectrum antibiotic therapy often is administered until the diagnosis is established, because bacterial shock is far more common than shock attributable to hantavirus.
Education for the Public
Environmental Control
Hantavirus infections of humans occur primarily in adults and are associated with domestic, occupational or leisure activities facilitating contact with infected rodents, usually in a building in a rural setting.
Eradicating the host reservoir is not feasible. Risk reduction includes practices that discourage rodents from colonizing the home and work environment and that minimize aerosolization and contact with rodent saliva and excreta. Tactics include eliminating food sources for rodents, reducing nesting sites by sealing holes and using “snap traps” and rodenticides. Before entering areas with potential rodent infestations, doors and windows should be opened to ventilate the enclosure. Regionally and culturally appropriate educational materials should be used to direct prevention messages.
Hantaviruses, because of their lipid envelope, are susceptible to diluted bleach solutions, detergents and most general household disinfectants. Dusty areas or articles should be moistened with 10% bleach or other disinfectant solution before being cleaned. Brooms and vacuum cleaners should not be used to clean rodent-infested areas. Use of a 10% bleach solution to disinfect dead rodents and wearing rubber gloves before handling trapped or dead rodents is recommended. Gloves and traps should be disinfected after use. The cleanup of areas potentially infested with hantavirus-infected rodents should be conducted by knowledgeable professionals using appropriate personal protective equipment. Potentially infected material should be handled according to local regulations for infectious waste.
Accessing Health Care
Persons who have been around rodents and subsequently develop symptoms of fever, deep muscle aches and severe shortness of breath should see their health care provider immediately. It is also important for these individuals to notify their health care provider that they have been around rodents — this will alert their physician to look closely for any rodent-carried disease, such as HPS.
For More Information
- Centers for Disease Control and Prevention – Hantavirus
- Nevada Office of State Epidemiology – Data & Statistics
Reporting of Possible Cases

References:
American Academy of Pediatrics. (2021). Hantavirus Pulmonary Syndrome. In. D. W. Kimberlin, E.D. Barnett, R Lynfield, and M.H. Sawyer (Eds.), Red Book: 2021 Report of the Committee on Infectious Diseases (pp. 354-357). Itasca, IL: American Academy of Pediatrics.
Centers for Disease Control and Prevention. (2023). Hantavirus. Retrieved from: https://www.cdc.gov/hantavirus/index.html.
Centers for Disease Control and Prevention. (2015) Hantavirus Infection, Non-Hantavirus Pulmonary Syndrome 2015 Case Definition. Retrieved from: https://ndc.services.cdc.gov/case- definitions/hantavirus-infection-non-hantavirus-pulmonary-syndrome-2015/.
Centers for Disease Control and Prevention. (2015) Hantavirus Pulmonary Syndrome (HPS) 2015 Case Definition. Retrieved from: https://ndc.services.cdc.gov/case-definitions/hantavirus-pulmonary- syndrome-2015/.
Washoe County Health District (2018). 2017 Annual Communicable Disease Summary. Retrieved from: https://nnph.org/files/ephp/communicable-diseases/annual-summary/CD_Annual_2017_FINAL.pdf.
Questions
For updated guidance, review the Division of Public and Behavioral Health Technical Bulletin webpage regularly. Email stateepi@health.nv.gov for other questions regarding hantavirus.

