CDC Provides New Laboratory Recommendations for Syphilis Testing
Introduction
Currently, no available nucleic acid amplification tests (NAATs) are cleared by Food and Drug Administration (FDA) for marketing in the United States to screen or diagnose syphilis, and culture for T. pallidum is cumbersome and is available only in selected research laboratories. Nontreponemal (lipoidal antigen) tests are most suitable for screening or diagnosis in conjunction with a medical history and physical examination when antibody titers are important to determine recent exposure to infection; a presumptive diagnosis in persons with signs or symptoms suggestive of syphilis; or to determine response to treatment.
Nontreponemal tests typically have been used as a screening test for syphilis; as a diagnostic test when patients have signs or symptoms suggestive of syphilis or have a known sexual contact when assessing possible reinfections; and when monitoring treatment outcomes.
When performed by an experienced laboratory technician and used in conjunction with treponemal tests, clinical history, physical examination and contact history, the nontreponemal (lipoidal antigen) tests are a highly reliable testing method for screening and determining the endpoint titer for subsequent serologic monitoring post-treatment.
The traditional algorithm for syphilis serologic screening begins with a nontreponemal test, and any reactive specimens are tested for confirmation by a treponemal test.
On Feb. 8, 2024, the Centers for Disease Control and Prevention (CDC) provided new evidence-based recommendations (see Table 1) for tests and methods that support a diagnosis of syphilis, including laboratory-based tests, point-of-care (POC) tests, processing of samples, and reporting of test results to aid laboratorians and clinicians in the detection of Treponema pallidum, the causative agent of syphilis. These recommendations are intended for use by clinical laboratory directors, laboratory staff, clinicians, and disease-control personnel who must choose among multiple available testing methods; establish standard operating procedures for collecting and processing specimens; interpret test results for laboratory reporting; and counsel and treat patients.
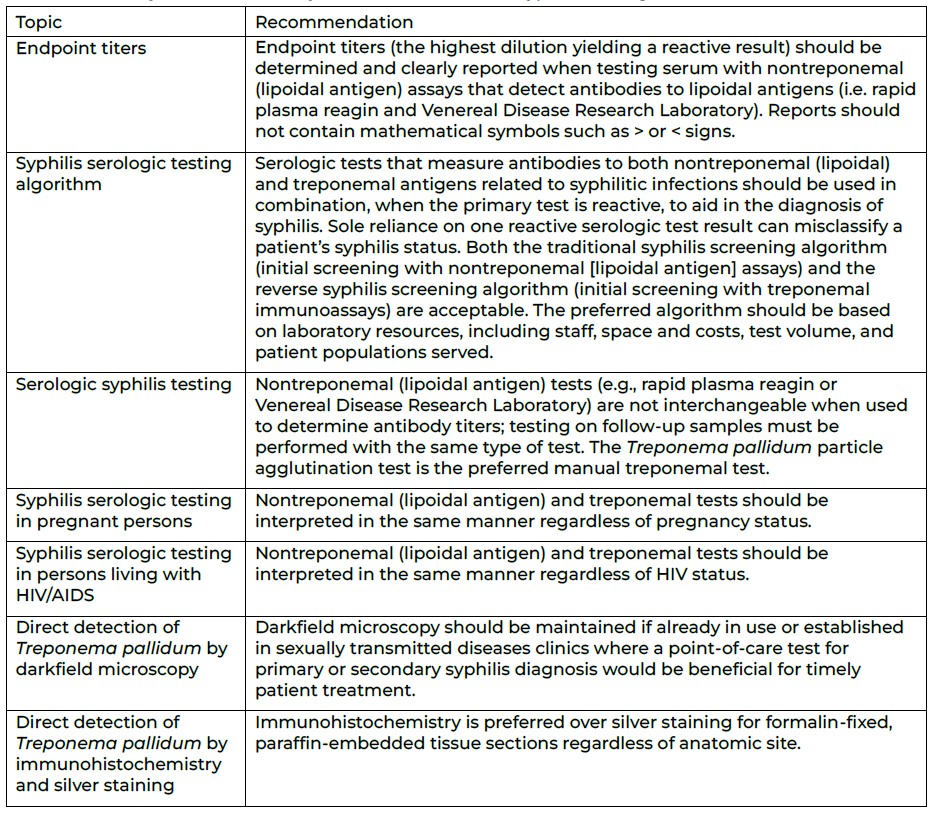
The primary recommendations fall under seven topics:
- Endpoint titers
- Syphilis serologic testing algorithm
- Serologic syphilis testing
- Syphilis serologic testing in pregnant persons
- Syphilis serologic testing in persons living with HIV/AIDS
- Direct detection of Treponema pallidum by darkfield microscopy
- Direct detection of Treponema pallidum by immunohistochemistry and silver
Table 1. Summary of CDC laboratory recommendations for syphilis testing, United States, 2024
Detailed CDC recommendations can be found here.
Reporting
All positive syphilis direct detection tests, along with specimen site and positive syphilis serologic tests, must be reported to the appropriate state or local public health authority (see table below). Laboratories should list all tests used, report each result with an interpretation, and document the syphilis algorithm applied to render the interpretation to both the ordering provider and the appropriate public health authority.

Reporting to health care providers
When reporting results to health care providers, laboratories should list all tests used, report each result with an interpretation, and document the syphilis algorithm applied to render the interpretation, when appropriate.
Questions
For updated guidance, review the Division of Public and Behavioral Health Technical Bulletin web page regularly. Email stateepi@health.nv.gov for other questions regarding the CDC laboratory recommendations for syphilis testing.

