Accelerated Testing and Surveillance for Avian Influenza A (H5N1) Human Cases
Background
Avian influenza A (H5N1) has been circulating widely among birds and mammals in the United States since 2022. In spring 2024, H5N1 was detected in dairy cattle for the first time. Since then, more than 900 affected dairy herds have been reported in at least 16 states. In December 2024, one dairy herd in Nye County, Nevada, tested positive for H5N1. H5N1 continues to affect poultry flocks nationwide, with the last poultry detection in Nevada reported in March 2023.
To date, more than 66 human cases of H5N1 have been reported in the United States. About 60% of these individuals had exposure to dairy herds, and about 35% had exposure to poultry farms (a few had no known animal exposures). California has declared a state of emergency due to the spread of bird flu, with the highest number of human cases reported. On December 13, the Centers for Disease Control and Prevention (CDC) confirmed the United States’ first severe H5N1 case in Louisiana, associated with backyard flocks, and this case was subsequently reported to be deceased on January 7.
Despite this, the CDC’s assessment of the immediate risk to the general public remains low. Nevada has not yet had a human case and has not declared a state of emergency at this time. No human-to-human transmission has been detected. Healthcare providers are urged to take the following actions to aid public health in ensuring rapid identification and response in suspected H5N1 cases in Nevada.
Actions for medical providers
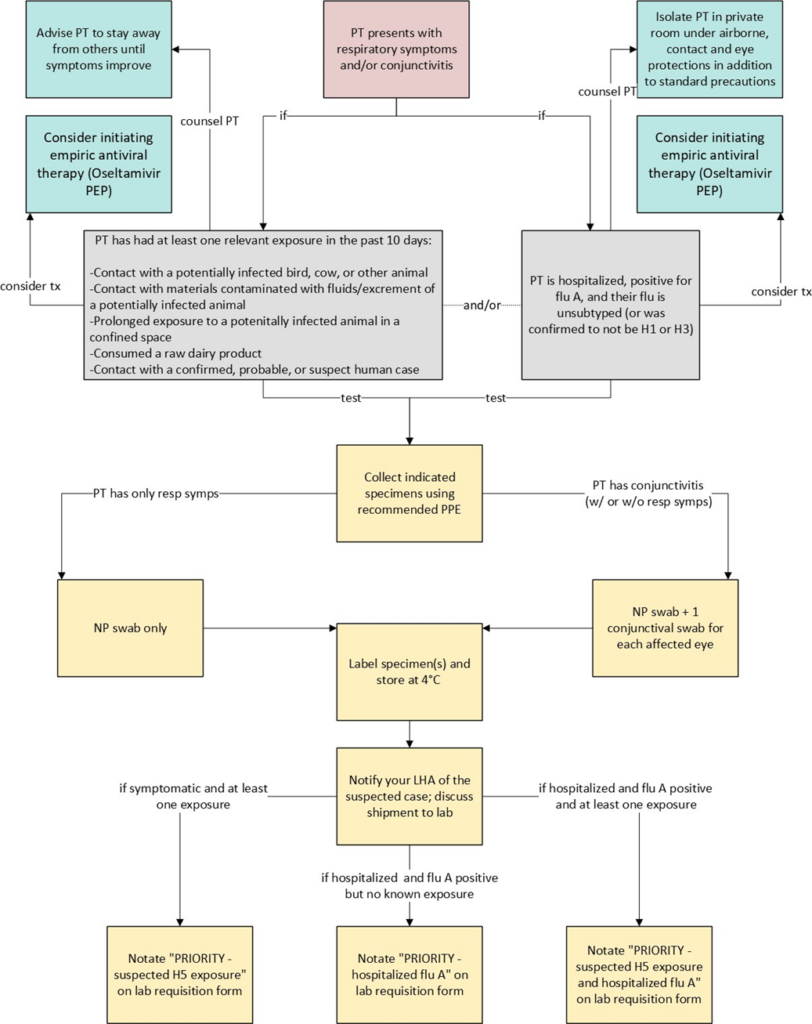
For an at-a-glance reference to the recommended clinical steps, please see the flowchart provided in Appendix A.
Evaluate for Influenza A (H5N1):
Consider Influenza A (H5N1) virus infection in patients who present with acute respiratory illness and/or conjunctivitis if they have had relevant exposure within the 10 days preceding symptom onset. Providers should thoroughly document relevant exposures in the patient’s medical record, including:
- Contact with potentially infected sick or dead birds, livestock, or other animals (e.g., handling, slaughtering, defeathering, butchering, culling).
- Consuming or preparing unpasteurized/raw milk or dairy products.
- Direct contact with water or materials contaminated with fluids or excrement from potentially infected animals.
- Prolonged exposure to potentially infected birds or other animals in confined spaces.
- Contact with a confirmed, probable, or suspect human case.
Special considerations for hospitalized patients:
All hospitalized patients with an unsubtyped positive influenza A test should have specimens subtyped for influenza A(H5) within 24 hours of influenza A confirmation at either a public health or clinical laboratory. Specimens confirmed as H1 or H3 are not required to undergo H5 subtyping.
Testing and specimen collection:
- DPBH recommends testing and the collection of appropriate specimens if your patient meets the above criteria (signs/symptoms plus an exposure).
- For all indicated patients: Collect 1 nasopharyngeal (NP) swab and place in its own tube of sterile viral transport medium (VTM).
- For indicated patients with conjunctivitis: Additionally, collect 1 conjunctival swab for each affected eye and place in sterile VTM. Swabs may be combined in one tube if two swabs are taken.
- Use Dacron or nylon-tipped swabs.
- Consider testing for other respiratory pathogens (including SARS-CoV-2 and RSV).
- Recommended personal protective equipment (PPE) during specimen collection includes an N95 respirator or equivalent, eye protection, and disposable gloves. While a disposable gown is not required, it is recommended.
- Label specimens and complete the laboratory requisition form for the designated laboratory, being sure to clearly notate “PRIORITY – suspected H5 exposure” OR “PRIORITY – hospitalized flu A” OR “PRIORITY – hospitalized flu A with suspected H5 exposure.”
- Place specimens immediately on refrigerant gel-packs or in 4°C refrigeration.
Notification and reporting:
If H5N1 infection is suspected, immediately establish two-way communication with the appropriate local health authority. The local health authority will coordinate with the designated public health laboratory to ensure the specimen can be received and tested within 24 hours of collection. If a public health laboratory is unavailable, specimens may be forwarded to a clinical laboratory after confirming the same testing capacity.
| Local Health Authority | Counties | Fax and Phone Number to Report |
| Carson City Health and Human Services (CCHHS) | Carson City, Douglas, and Lyon | Fax: 775-887-2138 Ph: 775-887-2190 (24 hours) |
| Central Nevada Health District (CNHD) | Churchill, Mineral, Eureka, and Pershing | Fax: 877-513-3442 Ph: 775-866-7535 (24 hours) |
| Northern Nevada Public Health (NNPH, formerly WCHD) | Washoe | Fax: 775-328-3764 Ph: 775-328-2447 (24 hours) |
| Southern Nevada Health District (SNHD) | Clark | Fax: 702-759-1414 Ph: 702-759-1300 (24 hours) |
| Nevada Division of Public and Behavioral Health (DBPH) Office of State Epidemiology | All other counties | Fax: 775-684-5999 Ph: 775-400-0333 (24 hours) |
Local health authorities should notify OSE the same day at dpbhepi@health.nv.gov pursuant to NAC 441a.180 and 441A.240.
Management and isolation:
- Strongly consider initiating empiric antiviral therapy. Oseltamivir Post-Exposure Prophylaxis (PEP), twice daily for five days, ideally initiated within 48 hours of symptom onset or within 48 hours of last- known exposure, can be given to persons who experienced high risk of exposure (without using recommended PPE) to animals confirmed to be infected or highly suspected to be infected with Influenza A(H5N1) virus. An unprotected exposure could also include breaches in or failures of recommended PPE.
- Advise symptomatic patients who are not hospitalized to isolate at home, away from household members, until at least 24 hours after symptoms have begun improving and patient is fever-free (temperature <100.4 °F (<38.0 °C) without the use of fever-reducing medications). Additionally, advise symptomatic patients to avoid work or school until avian influenza A(H5N1) has been ruled out.
- Hospitalized patients with suspected or confirmed influenza A(H5N1) should be isolated in a private room using airborne, contact, standard precautions, and eye protection.
Shipping and handling instructions
- Specimens should be stored refrigerated (2-8°C) and must be processed within 72 hours of collection.
- If testing cannot occur within 72 hours of collection, freeze the specimen at ≤-70°C.
- Residual specimens should be stored at ≤-70°C for potential further subtyping.
- Use triple packaging to transport specimens, maintaining a cold chain (2-4°C).
- Specimen handlers must follow safe handling practices and spill decontamination procedures.
Health care facilities and clinicians should coordinate specimen shipment with their Local Health Authority and the receiving laboratory. Specimen collection supplies such as swabs and viral transport medium may be requested in advance from the receiving laboratory.

Questions
For updated guidance, regularly review the Division of Public and Behavioral Health Technical Bulletin web page. For additional questions regarding avian influenza, email stateepi@health.nv.gov.
Appendix A