Updates to COVID-19 Laboratory Reporting Requirements
Background:
Entities in Nevada that have performed or analyzed a test intended to detect SARS-CoV-2 or to diagnose a possible case of COVID-19 have been mandated to report all results (positive, negative and inconclusive) to the Nevada Department of Health and Human Services (DHHS) Division of Public and Behavioral Health (DPBH). Due to recent updated guidance from the U.S. Department of Health and Human Services (HHS) and the Centers for Disease Control and Prevention (CDC), state guidance and reporting requirements are being updated to reflect the following changes, which will become effective April 4, 2022.i The updated guidance replaces the blanket requirement to report all SARS-CoV-2 test results and reflects more tailored reporting requirements that are specific to entity and test type. This updated policy change should help reduce the burden on clinical laboratories, point-of-care testing sites, and jurisdictions, while ensuring public health decision makers in Nevada continue to receive important testing data.
Updated requirements:
SARS-CoV-2 Nucleic Acid Amplification Test (NAAT) performed in a facility certified under Clinical Laboratory Improvement Amendments (CLIA) to perform moderate or high-complexity tests:
In accordance with the updated CDC requirements, CLIA-certified laboratories that are certified to perform moderate- or high-complexity testing must continue to report all test results (i.e. positive, negative, inconclusive) from NAAT testing (e.g., RT-PCR) to the appropriate public health authority in the same manner and through the same mechanisms that are currently in place.
This includes, but is not limited to, NAAT testing performed for SARS-CoV-2 by clinical laboratories, including public health, commercial, health care system, and academic laboratories.
All other SARS-CoV-2 testing (except antibody testing and self-administered tests):
Entities conducting all other SARS-COV-2 testing (e.g., testing conducted in a setting operating under a CLIA certificate of waiver, non-NAAT testing conducted in a facility certified under CLIA to perform moderate or high-complexity tests), must report positive test results. Reporting of negative results, either individual test results or in aggregate, is no longer required and therefore not reportable in Nevada. This includes rapid and/or point-of-care antigen and molecular (e.g., Abbott ID NOW, CUE Health) testing conducted in many settings (e.g., screening at schools, correctional facilities, employee testing programs, long-term care facilities, and rapid/point-of-care testing performed in pharmacies, medical provider offices, and drive-through and pop-up testing sites).
SARS-CoV-2 antibody testing and self-administered tests:
SARS-CoV-2 antibody testing performed in any setting is no longer reportable to the public health authority. SARS-CoV-2 self-administered tests are also not reportable to the public health authority.
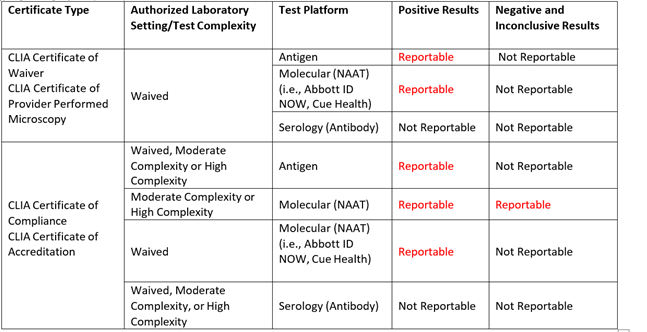
The table below gives an overview of the reporting requirements based on certificate type, test complexity and testing platforms starting April 4, 2022:
Overview of SARS CoV-2 Reporting Requirements by Certificate Type, Test Complexity, and Testing Platform (beginning April 4,2022):
Questions:
For updated guidance, please review the DPBH Technical Bulletin website and Nevada’s Health Response website regularly. Please email dpbhepi@health.nv.gov with questions.

