Moderna COVID-19 Vaccine Approved for Children and Adolescents 6-17 years
Background:
On June 14, 2022, the Vaccines and Related Biological Products Advisory Committee (VRBPAC) voted to recommend the authorization and use of the Moderna mRNA COVID-19 vaccine, for children and adolescents 6-17 years old, under their Emergency Use Authorization (EUA). On June 17, 2022, the U.S. Food and Drug Administration (FDA) authorized to amend the current EUA in place that allows the emergency use of the Moderna mRNA COVID-19 Vaccine for children and adolescents ages 6-17 years old to aid in the prevention of COVID-19.
On June 23, 2022, with the evidence presented on day two of the two-day Advisory Committee on Immunization Practices (ACIP) meeting, the committee voted unanimously to recommend a two-dose Moderna COVID-19 vaccine series for children ages 6-11 years and 12-17 years, under the EUA issued by FDA.
The Western States Scientific Safety Review Workgroup discussed data presented at the June 23, 2022, ACIP meeting and concluded, “… expanding the number of COVID-19 vaccines available for use in children and adolescents 6-17 years of age will allow them to more safely engage in educational and other activities important to their health and development; give parents a means of further protecting their children; and contribute to control of the COVID-19 pandemic in our states.” The Workgroup strongly urges that states, “… make every effort to reduce or eliminate disparities in the availability and uptake of COVID-19 vaccines in children and adolescents,” and notes this vaccine’s efficacy in the pre-Omicron era against symptomatic COVID-19 disease in these age groups. Therefore, the Workgroup supports the use of the Moderna COVID-19 vaccine under EUA as a two-dose primary series.
This technical bulletin summarizes the recent Moderna mRNA COVID-19 Vaccine eligibility recommended for children and adolescents ages 6-17 years old.
Those children and teens eligible to receive the recommended two-dose Moderna mRNA COVID-19 Vaccine primary series include:
- Any child ages 6 years to 11 years of age
- Dose interval: first dose administered on day zero, second dose administered 4-8 weeks after the first dose to complete the primary series
- Dose amount: 0.5mL/50 mcg each dose, to be administered intramuscularly
- A third primary series dose is approved for those children who have been determined to have certain kinds of immunocompromise. This third dose should be administered 28 days (4 weeks) after the second dose to complete the primary series.
- Any child/adolescent ages 12 years to 17 years of age
- Dose interval: first dose administered on day 0, second dose administered 4-8 weeks after the first dose to complete the primary series
- Dose amount: 0.5mL/100 mcg each dose, to be administered intramuscularly
- A third primary series dose is approved for those children who have been determined to have certain kinds of immunocompromise. This third dose should be administered 28 days (4 weeks) after the second dose to complete the primary series.
Moderna Immunization Schedule for Children 6 Months through 17 Years of Age
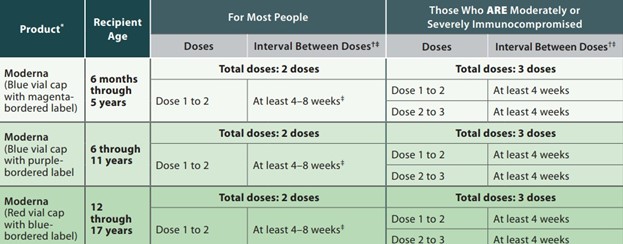
† Persons with a recent SARS-CoV-2 infection may consider delaying a primary series or booster dose by 3 months from symptom onset or positive test (if infection was asymptomatic).
‡ Some studies in adolescents and adults have shown the small risk of myocarditis associated with mRNA COVID-19 vaccines might be reduced and peak antibody responses and vaccine effectiveness may be increased with an interval longer than 4 weeks. An 8-week interval may be optimal for people who are not moderately or severely immunocompromised and ages 6 months–64 years.
The FDA evaluated and analyzed the effectiveness and safety data for the Moderna mRNA COVID-19 Vaccine through a rigorous and comprehensive evaluation process. This evaluation was used to support the EUA amendment for these pediatric populations. Effectiveness and safety data were generated in two ongoing, randomized, blinded, placebo- controlled clinical trials in the United States and Canada which enrolled children and adolescents between 6-17 years. To ensure ongoing safety monitoring, the FDA and the Centers for Disease Control and Prevention (CDC) have several systems in place to continually monitor COVID-19 vaccine safety and allow for the timely detection and investigation of potential safety concerns.
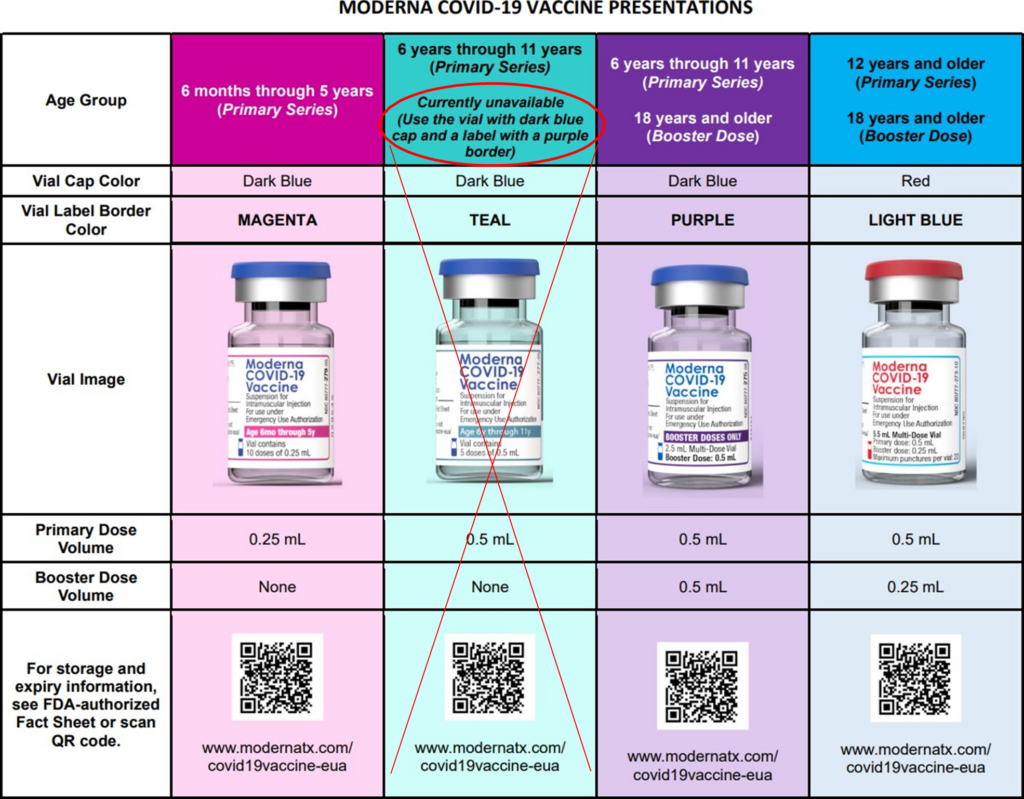
Moderna’s Vaccine Information Fact Sheets for the following ages have now been updated, in addition to a comprehensive wall chart. Below you will find additional information and resources for:
- Any Recipients and/or Caregivers and Healthcare Providers for those individuals ages 6 years to 11 years of age, in addition to a Letter to Healthcare Providers which includes important prescribing information.
- Any Recipients and/or Caregivers and Healthcare Providers for those individuals ages 12 years to 17 years of age
It is important to note the primary goal of the COVID-19 vaccine response should continue to be COVID-19 vaccine administration to the unvaccinated. COVID-19 vaccines may be co-administered with other routine age-appropriate vaccines at the same visit. The Nevada Department of Health and Human Services is encouraging families to speak with a health care provider about vaccination and COVID-19 vaccines. Families may be referred to NVCOVIDFighter.org or 800- 401-0946 for more information on vaccine access and other COVID-19 resources.
Questions:
For updated guidance, please review the DPBH Technical Bulletin web page and the Nevada Health Response website regularly. Email questions to dpbhcovid19vax@health.nv.gov.

