Novavax COVID-19 Vaccine, Adjuvanted, Now Recommended by CDC for 12+ Years
Background:
On July 13, 2022, the U.S. Food and Drug Administration (FDA) issued an Emergency Use Authorization (EUA) for the Novavax COVID-19 vaccine, Adjuvanted, in individuals 18 years of age and older for the prevention of COVID-19 caused by Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2).
The FDA has determined that the Novavax COVID-19 vaccine has met the statutory criteria to be issued an Emergency Use Authorization (EUA). By authorizing an additional COVID-19 vaccine, adults in the United States who have not yet received a COVID-19 vaccine now have expansion in available vaccine options. This vaccine contains the SARS-CoV-2 spike protein and Matrix-M adjuvant. Adjuvants are ingredients used in some vaccines that help to create a stronger immune response for the vaccinated individual.
On July 19, 2022, with the evidence presented at the Advisory Committee on Immunization Practices (ACIP) meeting, the committee voted unanimously to recommend a two-dose Novavax COVID-19, Adjuvanted vaccine series for persons 18 years and older, under the EUA issued by FDA.
On August 19, 2022, the U.S. Food and Drug Administration extended and reissued Emergency Use Authorization (EUA) for the Novavax COVID-19 vaccine to individuals 12 through 17 years of age. On August 22, 2022, the Centers for Disease and Prevention signed a decision memo that Novavax COVID-19 vaccine, Adjuvanted, be used as another primary series option for adolescents ages 12 through 17.
This technical bulletin summarizes the recent Novavax COVID-19 vaccine eligibility recommended for individuals ages 12 years of age and older. At this time, the Novavax COVID-19 vaccine is only authorized to be administered as a primary series and is not authorized to be administered as a booster dose. Upon completion of a Novavax primary series, individuals 12 years of age and older will be eligible to receive a COVID-19 bivalent booster dose (administered 2 months or more after the last primary series dose). To determine bivalent booster dose eligibility and primary series dose completion, please use CDC’s Interim COVID-19 Vaccination Schedule.
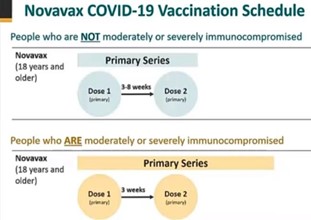
Those adolescents and adults eligible to receive the recommended two-dose Novavax COVID-19 Vaccine primary series include:
- Any individual 12 years of age or older who is not moderately or severely Immunocompromised
- Dose interval: first dose administered on day 0, second dose administered 3-8 weeks after the first dose to complete the primary series.
- Dose amount: 0.5 mL each dose (containing 5 mcg SARS-CoV-2rS and 50 mcg Matrix-M adjuvant) to be administered intramuscularly.
- Any individual 12 years of age or older who is moderately or severely Immunocompromised
- Dose interval: first dose administered on day 0, second dose administered 3 weeks after the first dose to complete the primary series.
- Dose amount: 0.5 mL each dose (containing 5 mcg SARS-CoV-2rS and 50 mcg Matrix-M adjuvant) to be administered intramuscularly.
** According to the CDC, an 8-week interval between the first and second primary series doses of Moderna, Novavax, and Pfizer-BioNTech COVID-19 vaccines may be optimal for some people ages 6 months–64 years, especially for males ages 12–39 years, as it may reduce the small risk of myocarditis/pericarditis associated with these vaccines. A shorter interval (3 weeks for Novavax and Pfizer-BioNTech; 4 weeks for Moderna) between the first and second doses remains the recommended interval for people who are moderately or severely immunocompromised; adults ages 65 years and older; and in situations in which there is increased concern about COVID-19 community levels or an individual’s higher risk of severe disease.
It is important to note the Storage and Handling requirements of this vaccine. The Novavax COVID-19 vaccine may not be kept frozen and does not use diluent. Unpunctured, multi-dose vials must be stored in a refrigerator between 2 degrees to 8 degrees Celsius (36 degrees to 46 degrees Fahrenheit). After the first needle puncture, hold the vial between 2 degrees to 25 degrees Celsius (36 degrees to 77 degrees Fahrenheit) for up to 6 hours. Discard the vial 6 hours after the first puncture.
Note that there is no expiration date printed on any Novavax COVID-19 vaccine vials and/or cartons. To find the expiration date, access the Novavax COVID-19 Vaccine website, navigate to the “United States Healthcare Professional” section of the website, and enter the lot number printed on the carton or vial into the “Expiry Date Checker” tool. Providers must track the expiration date of each vial and should use CDC’s COVID-19 Vaccine Expiration Date Tracking Tool for tracking this information.


The FDA evaluated and analyzed the effectiveness and safety data for the Novavax COVID-19 vaccine through a rigorous and comprehensive evaluation process. This evaluation was used to support the issuance of an EUA. Effectiveness and safety data were generated in an ongoing, randomized, blinded, placebo-controlled study in the United States and Mexico which enrolled participants 18 years of age and older who did not have evidence of SARS-CoV-2 infection through six days after receiving the second vaccine dose. To ensure ongoing safety monitoring, the FDA and the Centers for Disease Control and Prevention (CDC) have several systems in place to continually monitor COVID-19 vaccine safety and allow for the timely detection and investigation of potential safety concerns.
Novavax’s COVID-19 Vaccine, Adjuvanted Information Fact Sheets have now been updated to reflect the new adolescent authorization. Below you will find additional information and resources for:
- Any Recipients and/or Caregivers and Healthcare Providers for those individuals 12 years of age and older.
It is important to note the primary goal of the COVID-19 vaccine response should continue to be COVID-19 vaccine administration to the unvaccinated. The Nevada Department of Health and Human Services is encouraging individuals to speak with a health care provider about vaccination and COVID-19 vaccines. Individuals may be referred to NVCOVIDFighter.org or 1-800-401-0946 for more information on vaccine access and other COVID-19 resources.
Questions:
For updated guidance, review the DPBH Technical Bulletin web page and the Nevada Health Response website regularly. Email questions to dpbhcovid19vax@health.nv.gov.

