Novavax COVID-19 Vaccine, Adjuvanted Booster Now Authorized for 18+
Background
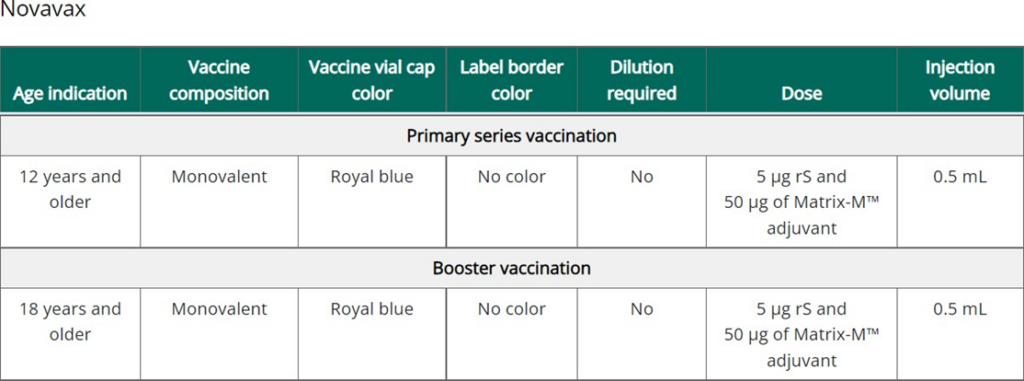
On October 19, 2022, the U.S Food and Drug Administration re-issued a letter of authorization, in its entirety, to authorize the use of Novavax COVID19 Vaccine, Adjuvanted as a first booster dose. By authorizing an additional COVID- 19 booster vaccine, adults in the United States who have not yet received a COVID-19 booster vaccine, now have expansion in available COVID-19 booster vaccine options. This monovalent booster vaccine contains the SARS-CoV-2 spike protein and Matrix-M adjuvant. Adjuvants are ingredients used in some vaccines that help to create a stronger immune response for the vaccinated individual.
This technical bulletin summarizes the recent Novavax COVID-19 Vaccine, Adjuvanted booster eligibility recommended for individuals ages 18 years of age and older. At this time, Novavax COVID-19 Vaccine, Adjuvanted has been authorized as a first booster dose only. If an individual has received anything more than the doses required to complete their primary series, then they are not eligible to receive a Novavax booster. To be eligible, an individual must not have received a prior monovalent or bivalent COVID-19 booster of any brand.
This booster dose is for the following individuals, at least 6 months after completion of a primary vaccination with a FDA authorized COVID-19 vaccine (Moderna, Pfizer, Novavax or Janssen) and who have not yet received a booster of any formulation:
- Individuals 18 years of age and older for whom an FDA-authorized mRNA bivalent COVID-19 booster vaccine (either Moderna or Pfizer) is not accessible or clinically appropriate.
- Individuals 18 years of age and older who elect to receive the Novavax COVID-19 Vaccine, Adjuvanted because they would otherwise not receive a booster dose of a COVID-19 vaccine.
Novavax’s COVID-19 Vaccine, Adjuvanted Information Fact Sheets have now been updated to reflect their new monovalent booster authorization. Novavax’s COVID-19 Vaccine, Adjuvanted Information Fact Sheets have now been updated to reflect their new monovalent booster authorization. Below you will find additional information and resources for:
- Any Recipients and/or Caregivers and Healthcare Providers for those individuals 18 years of age and older.
- Storage & Handling: The same storage and handling requirements and guidelines as all other Novavax COVID-19 adjuvanted vaccines.
For more information and/or additional resources, please visit the Centers for Disease Control and Prevention (CDC)’s COVID-19 Interim Clinical Considerations website.
It is important to note the primary goal of the COVID-19 vaccine response should continue to be COVID-19 vaccine administration to the unvaccinated. The Nevada Department of Health and Human Services is encouraging individuals to speak with a health care provider about vaccination and COVID-19 vaccines. Individuals may be referred to NVCOVIDFighter.org or 1-800-401-0946 for more information on vaccine access and other COVID-19 resources.
Questions:
For updated guidance, please review the DPBH Technical Bulletin web page and the Nevada Health Response website regularly. Email questions to dpbhcovid19vax@health.nv.gov.

