Laboratory Reporting Negative HIV and HEP C Tests
Summary
On Dec. 10, 2023, the Nevada Legislative Commission approved amendments to the Nevada Administrative Code (NAC) 441A that updated reporting requirements for both hepatitis C virus (HCV) and human immunodeficiency virus (HIV). Official codification of NAC 441A is pending, however the approved amendments can be found online here.
It is now required that the director or other person in charge of a medical laboratory report to the health authority both positive and negative results of any test or examination conducted by the medical laboratory for HCV and HIV. Reporting must occur in the manner provided in NAC 441A.225.
Such a report must include, without limitation:
- The date and result of the test or examination.
- The name, address, and if available, telephone number of the person from whom the specimen was obtained.
- If available, the sex, age, and date of birth of the person from whom the specimen was obtained.
- The name of the health care provider who ordered the test or examination.
- The name and address or telephone number of the medical laboratory.
- Any other information requested by the health authority, if available.
Laboratory Reporting
Laboratories with HL7 capability should plan to report laboratory results through the electronic laboratory reporting (ELR) system. The Nevada Division of Public and Behavioral Health (DPBH), Office of State Epidemiology (OSE) will assist in onboarding laboratories and testing entities and can be reached at dpbhelronboarding@health.nv.gov to start the process. OSE will provide instructions on the best alternative mechanism to report for entities without HL7 capability.
Provider Reporting
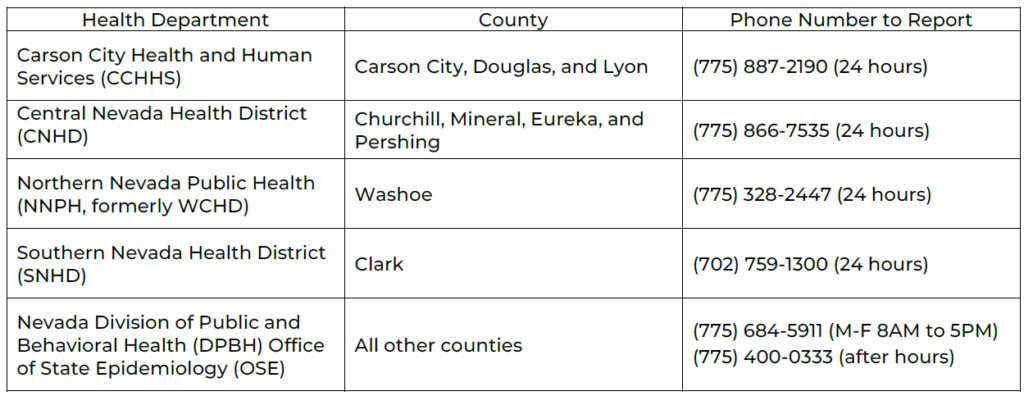
Nevada’s Confidential Morbidity Report Form can be found online here.
References and Resources
2022 – 2026 Nevada HIV Integrated Prevention and Care Plan. (2022, October). End HIV Nevada. https://endhivnevada.org/integrated-plan/
2023 Nevada State Board of Health. (n.d.). https://dpbh.nv.gov/Boards/BOH/Meetings/2023/2023_Nevada_State_Board_of_Health/
Hepatitis C | Centers for Disease Control and Prevention. (n.d.). https://www.cdc.gov/hepatitis/hcv/index.htm
HIV Testing | Centers for Disease Control and Prevention. (n.d.). https://www.cdc.gov/hiv/testing/index.html
Nevada Legislative Commission. (n.d.). https://www.leg.state.nv.us/App/InterimCommittee/REL/Interim2023/Meetings
State of Nevada. (n.d.). 2021 Assembly Bill 192. https://www.leg.state.nv.us/App/NELIS/REL/81st2021/Bill/7583/Overview
State Of Nevada. (n.d.). 2021 Senate Bill 275. https://www.leg.state.nv.us/App/NELIS/REL/81st2021/Bill/7864/Overview
The State of Nevada, Ending the HIV Epidemic Plan. (2020, December). End HIV Nevada. https://endhivnevada.org/ending-the-hiv-epidemic/
Questions
For updated guidance, review the Division of Public and Behavioral Health Technical Bulletin web page regularly. Email questions to stateepi@health.nv.gov.

